Background
Multiple myeloma (MM) is the second most common hematologic malignancy and is associated with significant patient burden. Renal impairment has been shown to affect up to 50% of patients (pts) with MM. Renal impairment is an independent predictor of poor survival outcomes for pts with MM, with a median survival of approximately half that of pts without renal impairment.
Aims
To assess change in renal function by treatment line and drug class among pts with MM and renal impairment (defined as eGFR <50 mL/min/1.73 m2 using the Modification of Diet in Renal Disease equation [eGFR-MDRD]), and to assess real-world outcomes by baseline renal status, renal response, and drug class.
Methods
Using the nationwide Flatiron Health EHR-derived de-identified database based in the United States, we identified MM pts diagnosed and treated between January 2011 and November 2019 who received ≥1 line of MM therapy and had information on race. We assessed the distribution of pts by eGFR-MDRD level at the start of the first and second line of therapy and the distribution of therapy use (ie, proteasome inhibitor [PI], immunomodulatory drug [IMiD], monoclonal antibody [mAb]). We also evaluated overall survival (OS) by treatment line, stratified by eGFR at the start of each treatment line. Complete renal response (CRR) was assessed in pts with eGFR-MDRD <50 mL/min/1.73 m2; using International Myeloma Working Group recommendations, responders were defined as pts with ≥1 eGFR measurement ≥60 mL/min/1.73 m2 during the treatment line. Logistic regression models were used to examine the association between treatment class and complete renal responder status adjusted for other treatment classes received, age, sex, race, practice type, year of therapy line, and cytogenetic risk. Cox proportional hazard models were used to examine OS by treatment and renal response, adjusted for other treatment classes received, age, sex, race, practice type, year of therapy line, and cytogenetic risk. Flatiron Health, Inc., did not participate in the analysis of this data.
Results
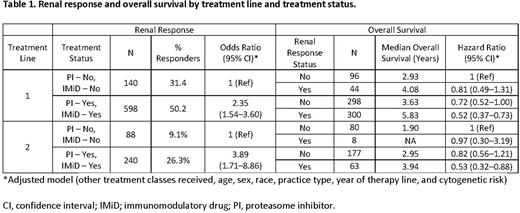
For the 6990 pts included in the analysis, the mean age at start of initial therapy was 68 years, 46% were female, and 17% were Black. At start of treatment lines 1 and 2, approximately 25% had eGFR-MDRD <50 mL/min/1.73 m2. At treatment initiation, pts with renal impairment were older (71 vs 67 years) and were more likely to present with ISS stage III at diagnosis (38% vs 10%). Pts with renal impairment at the start of each treatment line exhibited decreased OS. Among pts with renal impairment, pts with PI use in first (adjusted odds ratio [aOR] 1.36; 95% CI, 1.04-1.77) or second line (aOR 2.09; 95% CI, 1.38-3.16) were significantly more likely to have a CRR than those without PI use. Pts with IMiD use in first (aOR 2.14; 95% CI, 1.68-2.72) and second (aOR 1.61; 95% CI, 1.10-2.36) line were significantly more likely to have a CRR than those without IMiD use. When classified by both PI and IMiD use, pts with both PI and IMiD use were significantly more likely to have a CRR than those without use of either in the first (aOR 2.35; 95% CI, 1.54-3.60) and second line (aOR 3.89; 95% CI, 1.71-8.86). Pts with PI and IMiD use as well as a CRR also had greater OS in the first (adjusted hazard ratio [aHR] 0.52; 95% CI, 0.37-0.73) and second line (aHR 0.53; 95% CI, 0.32-0.88) vs pts without either PI or IMiD use and no CRR. The use of available mAbs in line 1 or line 2 was not significantly associated with renal response. Lower mAb use, especially in earlier treatment lines, prevented further analyses by mAb combination therapies.
Conclusion
In this study, MM pts with renal impairment were more likely to be older and to present with ISS stage III at diagnosis. Pts with decreased renal function exhibited decreased OS compared with non-renally impaired pts. Pts who were treated with combination therapy that included PIs and IMiDs together in early treatment lines were more likely to have a CRR and OS was prolonged among these pts, highlighting the benefits of combination therapy. These data suggest that treatment inducing a renal response may result in improved outcomes. Future investigations with larger datasets may improve the understanding of the prognostic value of renal impairment and optimal combination treatment regimens for these pts.
Mikhael:Amgen, Celgene, GSK, Janssen, Karyopharm, Sanofi, Takeda: Honoraria. Singh:Sanofi-Genzyme: Current Employment. Rice:Sanofi: Current Employment.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal